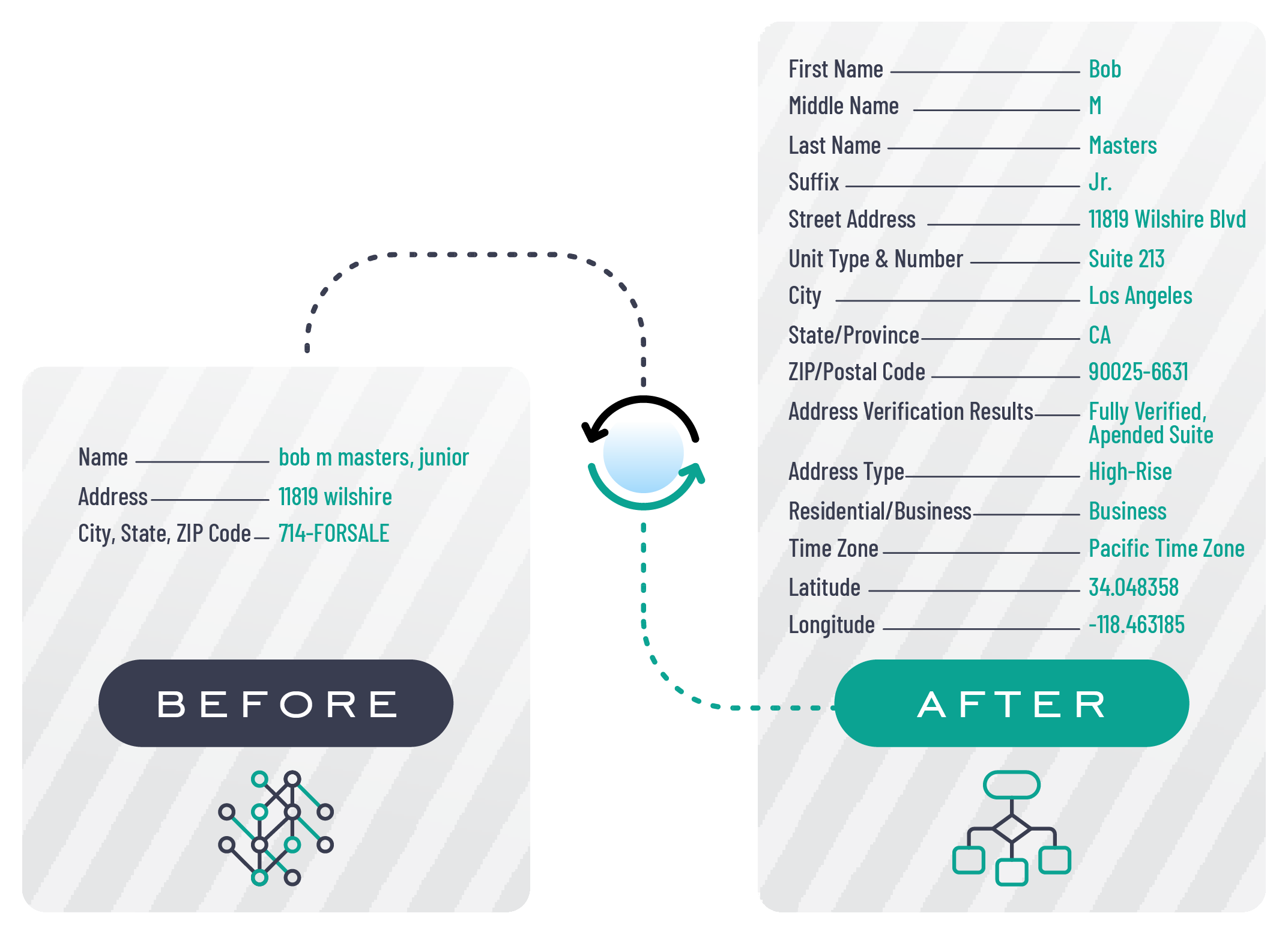
In today's digital age, data plays a crucial role in decision-making, marketing strategies, and overall business success. However, with the vast amount of data available, ensuring its quality and accuracy can be a challenging task. This is where data enrichment services come into play. Data enrichment services provide businesses with the tools and resources they need to enhance their data quality and accuracy, ultimately leading to better insights and improved outcomes. You can also hop over this website to find the best data enrichment services.
The Importance of Data Quality
Why is data quality important?
- High-quality data ensures accurate analysis and decision-making.
- Poor data quality can lead to costly mistakes and missed opportunities.
- Quality data enhances customer experiences and relationships.
- It improves the overall efficiency and effectiveness of business operations.
Challenges of maintaining data quality
Despite the importance of data quality, many businesses face challenges in maintaining it. Some common challenges include:
- Outdated or incomplete data sets
- Duplicate records
- Inconsistent data formats
- Lack of standardized data entry processes
What are Data Enrichment Services?
Data enrichment services involve the process of enhancing existing data sets with additional information to improve their quality, accuracy, and completeness. By leveraging various data sources and technologies, data enrichment services help businesses fill in the gaps in their data and ensure it is up-to-date and reliable.
Key features of data enrichment services
- Appending missing information to existing data records
- Standardizing data formats and structures
- Removing duplicate records
- Verifying and validating data accuracy
- Enhancing data with additional attributes and insights
Benefits of Data Enrichment Services
Improved Data Quality
- Enhanced accuracy and completeness of data
- Reduction of errors and inconsistencies
- Up-to-date and reliable data sets
Better Decision-Making
- Access to more relevant and comprehensive data
- Insights-driven decision-making processes
- Increased confidence in strategic initiatives
Enhanced Customer Relationships
- Personalized customer experiences based on enriched data
- Improved targeting and segmentation strategies
- Increased customer satisfaction and loyalty
Choosing the Right Data Enrichment Service Provider
When selecting a data enrichment service provider, it is essential to consider the following factors:
Data Sources and Accuracy
- Ensure the provider has access to reliable and diverse data sources.
- Verify the accuracy and freshness of the data being used for enrichment.
Customization and Scalability
- Look for a provider that offers customizable enrichment solutions tailored to your specific needs.
- Scalability is crucial to accommodate future growth and evolving data requirements.
Security and Compliance
- Ensure the provider follows strict security protocols to protect your data.
- Compliance with data privacy regulations such as GDPR and CCPA is essential.
Conclusion
Data enrichment services are instrumental in helping businesses improve the quality and accuracy of their data, leading to better decision-making, enhanced customer relationships, and overall operational efficiency. By leveraging data enrichment services, businesses can stay ahead of the competition and unlock new opportunities for growth and success.








